
Conclusionįrom the above points, we can have the essence of the beauty of carbon. Other metallic carbides have significant purposes as hotness resistants and metal cutters. Calcium carbide is utilized to get ready acetylene it’s utilized for welding and cutting metals, as well as concerning readiness of other natural mixtures. Carbon tetrachloride and carbon disulfide are significant modern solvents. Carbon monoxide is utilized as a decrease specialist in numerous metallurgic cycles. Carbon dioxide is utilized in drinks carbonation, in fire dousers, and, in a strong state, as a cooler (dry ice). Vegetal carbon, a nebulous type of carbon, is utilized as a gas permeable and blanching specialist. One more carbon structure, the graphite, is utilized for high-temperature cauldrons, dry cell, and light curve cathodes, for pencil tips, and as an oil. ApplicationsĬarbon has a ton of purposes, including adornment motivations behind precious stones in gems or dark smoke color in auto’s edges and printer’s ink. A nucleic acid is a polymeric macromolecule comprised of rehashed units of monomeric ‘nucleotides’ made out of a nitrogenous heterocyclic base which is either a purine or pyrimidine, a pentose (five-carbon) sugar (either ribose or 2′-deoxyribose), and one to three phosphate gatherings. Amino acids contain carbon, hydrogen, nitrogen, and oxygen. Cholesterol’s atomic recipe is C 27 H 46 O. Cholesterol has a mind-boggling design of four connected hydrocarbon rings and a hydrocarbon tail. The cholesterol that you endeavor to keep inside specific cutoff points is an illustration of a lipid.Ĭholesterol is one of the primary parts of creature cell layers and permits the cell to be adaptable and change shape. Lipids generally alluded to as fats are likewise polymers. Sucrose is one polymer of the glucose monomer. So basically, polymers are buildings of monomers. The underlying units that makeup polymers are called monomers. Polymers are buildings of rehashed underlying units. All mind-boggling natural atoms, similar to sucrose, are known as polymers. Sucrose has the atomic recipe C 12 H 22 O 11. An illustration of carb is table sugar, known as sucrose. Starches are particles made of carbon, hydrogen, and oxygen. In straightforward natural mixtures, carbon is clung to simply hydrogen.Ĭomplex natural mixtures, particularly those engaged with living frameworks, have carbons clung to at minimum hydrogen and oxygen. These are the most mind-boggling kinds of natural mixtures. The four principle gatherings of carbon particles of life are proteins, starches, lipids, and nucleic acids. This flexible formation gives rise to various complex organic compounds. The principle justification for the worth of carbon is that it can frame four bonds all at once, which permits it to shape perplexing, adaptable atoms. The various physical properties of carbon are as follows: –ġ) Colour: It is black or dull grey in coloring.ģ) Allotropes: It has several allotropes. ġ3) Energy of third ionisation: The energy of the third ionisation of carbon is 4618.8 KJ mol -1. ġ2) Energy of second ionisation: The energy of the second ionisation of carbon is 2351.9 KJ mol -1. ġ1) Energy of first ionisation: The energy of first ionisation of carbon is 1086.1 KJ mol -1.
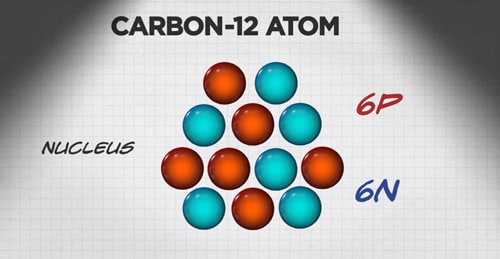
ģ) Electronegativity: According to Pauling, the electronegativity of carbon is 2.5.Ĥ) Boiling Point: Its boiling point is 4827 o C.ĥ) Melting Point: Its melting point is 3652 o C.Ħ) Density: Its density is 2.2 g cm -3 at 20 o C.ħ) Ionic Radius: The ionic radius for C 4- is 0.26 nm and for C 4+ is 0.015 nm.Ĩ) Van Der Waals Radius: Its Van Der Waals radius is 0.091 nm.ġ0) Electronic Shell: The electron shell configuration of carbon is 2s 2 2p 2. The various chemical properties of carbon are as follows: –ġ) Atomic Number: The atomic number of carbons is 6.Ģ) Atomic Mass: Its atomic mass is 12.011 g mol -1. In short, the uniqueness majorly comes from the chemical properties like electronegativity of carbon and its physical properties add a cherry on it. It is the second most plentiful component in the human body by mass (around 18.5%) after oxygen. Its overflow, its exceptional variety of natural mixtures, and its strange capacity to shape polymers at the temperatures normally experienced on Earth empower this component to act as a typical component of all known life. It is the fifteenth most plentiful component in the Earth’s hull, and the fourth most bountiful component in the universe by mass after hydrogen, helium, and oxygen. It is one of only a handful of exceptional components known since antiquity. Carbon is derived from the Latin root word “Carbo” meaning “Coal”.


 0 kommentar(er)
0 kommentar(er)
